Podosomes are actin-rich adhesion and invasion structures. Especially in macrophages, podosomes exist in two subpopulations, large precursors at the cell periphery and smaller podosomes (successors) in the cell interior. The mechanisms that differentially regulate these subpopulations are largely unknown. We could show that the membrane- and cytoskeleton-associated protein supervillin localizes preferentially to successor podosomes, and is recruited to precursors only upon their dissolution (Bhuwania et al., J. Cell Sci, 2012).
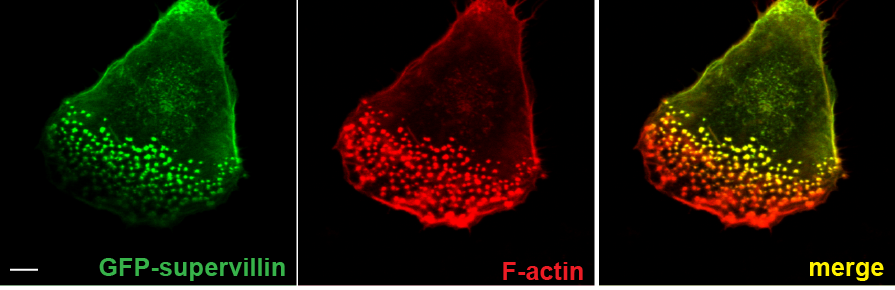 Supervillin localizes preferentially to successor podosomes.
Confocal immunofluorescence micrographs of 7 day cultured primary human macrophage expressing GFP–SV (green) and stained for F-actin (red); merged image shown (right). Note absence of GFP–SV from precursors (cell periphery of migratory cell or leading edge of polarized cell). Bar: 10 μm |
|
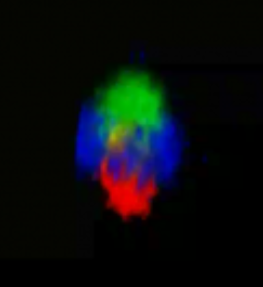 Supervillin forms a cap-like structure on top of the podosome core.
3D reconstruction of z-stacks of a single podosome. Cap-like distribution of GFP–SV (green) on top of the F-actin–rich podosome core (red), surrounded by a ring of vinculin (blue). To see movie, click on the image. (sequence 17 sec; 474 KB) |
|
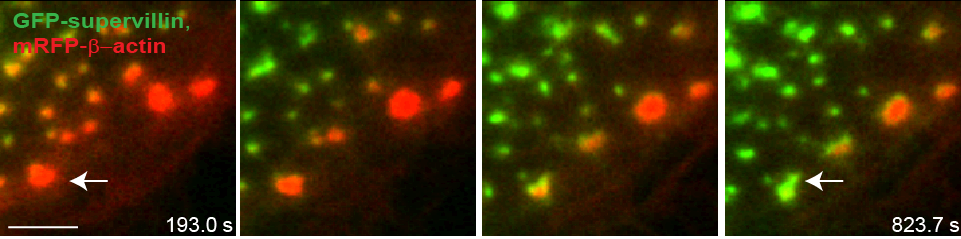 Precursors acquire supervillin upon their dissolution.
Still images from confocal time-lapse video of a macrophage expressing mRFP–β-actin (red) and GFP–SV (green) Note absence of GFP–SV from large podosome precursors at the leading edge (lower right). Note the appearance of supervillin at precursors concomitant with the dissolution of the F-actin signal, as the cell begins to withdraw. Bar: 5 μm |
|
In live-cell imaging, small accumulations of GFP–myosin-IIA often appeare in proximity to mRFP-tagged supervillin at successor podosomes and importantly, the appearance of these myosin IIA accumulations often precedes or coincides with podosome dissolution. These observations suggest supervillin at successor podosomes recruits and/or activates myosin IIA, causing the dissolution of these structures. In addition, we find that supervillin binds only contractile myosin. Moreover, supervillin itself can induce myosin contractility, which probably leads to localized feedback loops of myosin activation resulting in podosomedissolution.
 GFP-myosin IIA contacts dissolving podosomes.
Confocal time-lapse video of a macrophage expressing GFP-myosin IIA (green) and mRFP-supervillin (red). Note formation of GFP-myosin IIA-enriched trailing edge and absence of mRFP-supervillin from podosomes at the leading edge (lower right). Contact of GFP-myosin IIA with mRFP-positive podosomes at the rear of the podosome field precedes their disappearance, while podosomes at the forward side of the field acquire mRFP-supervillin. To see movie, click on the image. (sequence 7 sec; 88.4 KB) |
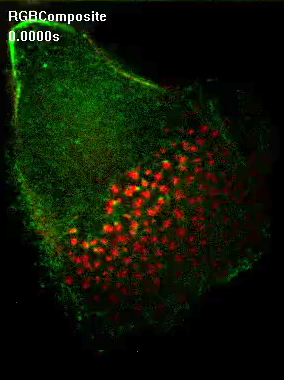
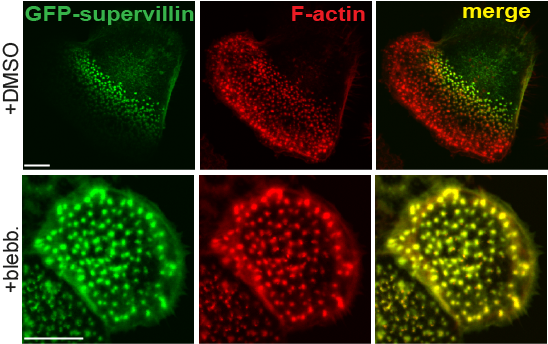 Differential localization of supervillin to successor podosomes requires contractile myosin.
Confocal images of macrophages overexpressing GFP supervillin and treated with DMSO as a control (first panel) or myosin II inhibitor blebbistatin (second panel).Note loss of differential localization of supervillin upon myosin inhbition. |
|
Collectively, we could show that podosome subpopulations differ in their molecular composition and identify supervillin, in cooperation with myosin IIA, as a crucial factor in the regulation of podosome turnover and function.
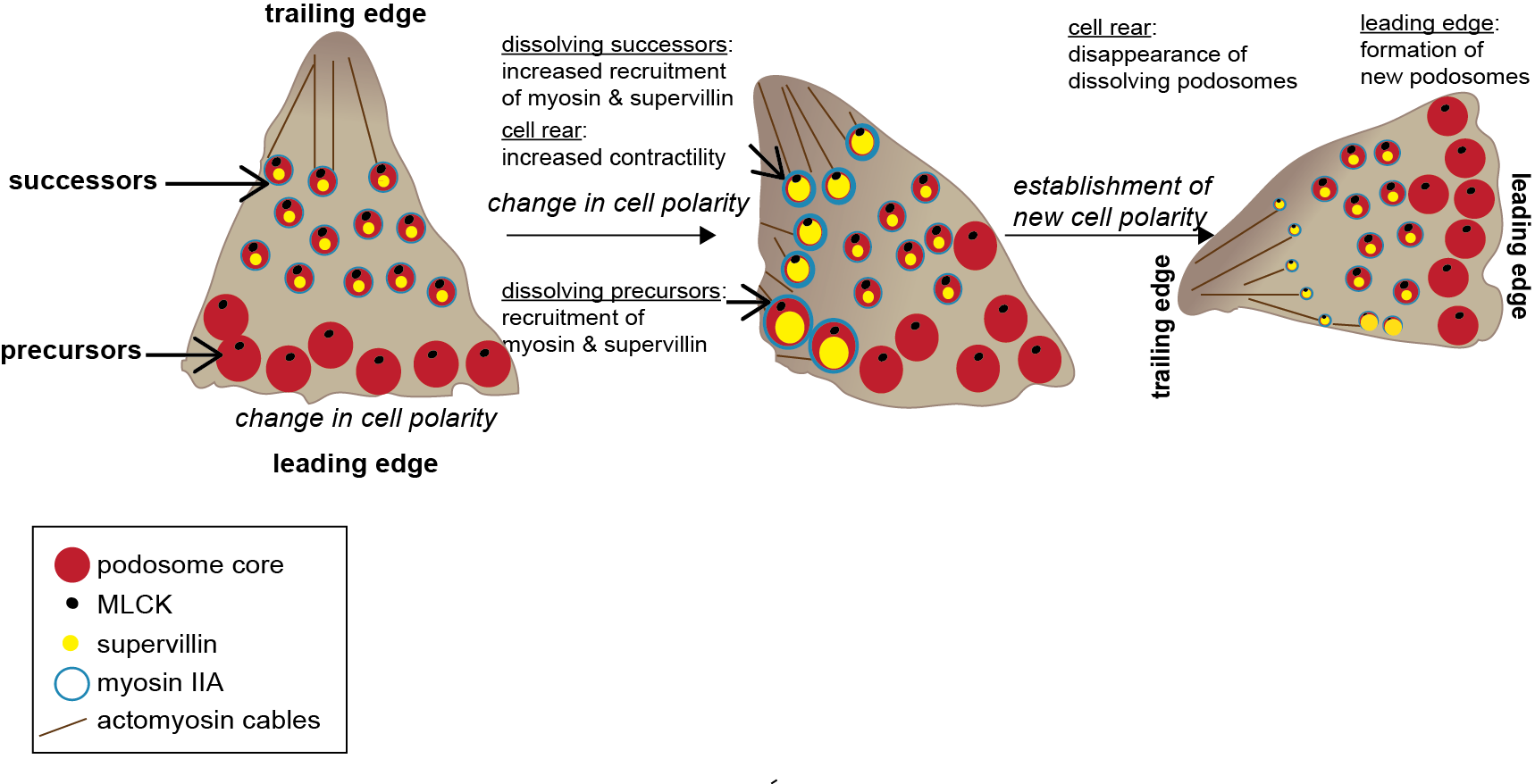
Model of supervillin- and myosin-IIA-regulated turnover of podosomes
Bhuwania et al., J. Cell Sci, 2012






