Borrelia burgdorferi – the causative agent of Lyme disease
|
Spirochetes of the Borrelia burgdorferi sensu lato complex are the causative agent of Lyme borreliosis, a chronic multisystemic disorder affecting primarily the skin, nervous system, heart and joints. Borreliae are transmitted by a tick bite and disseminate through the blood stream to various organs.
In order to eliminate invading borreliae, professional phagocytes such as macrophages and dendritic cells are recruited to the site of infection. Uptake and subsequent degradation of the spirochetes is thus of critical importance for the prevention of borreliae dissemination and the development of Lyme disease. |
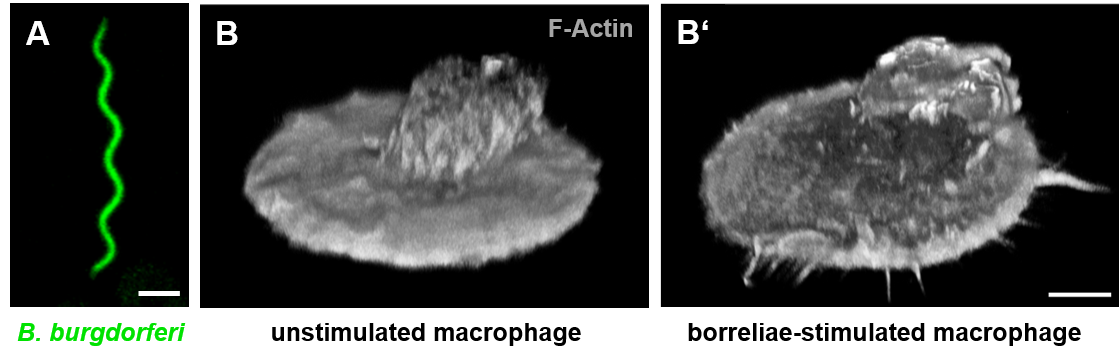
Borrelia burgdorferi sensu stricto induce filopodia formation in human macrophages.
(A) Confocal micrograph of GFP-expressing Borrelia burgdorferi. Scale bar: 2 µm. (B+B') Z-stack of a primary macropage (stained for F-actin) without stimulation (B) or after 1h of coincubation with Borrelia burgdorferi (B'). Scale bar: 5 µm. Upon stimulation with borreliae, macrophages form filopodial protrusions that arise from the cortical network. Click image to enlarge
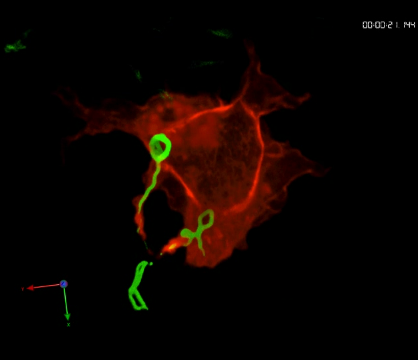
Phagocytosis of borreliae by primary human macrophage.
Time-lapse movie of confocal z-stack showing a primary human macrophage expressing RFP-Lifeact (red) internalizing several GFP-expressing spirochetes (green) with actin-rich cell protrusions. To see movie, click on the image. (sequence 41 min; 4.4 MB)
Formins regulate coiling phagocytosis of Borrelia burgdorferi by primary human macrophages
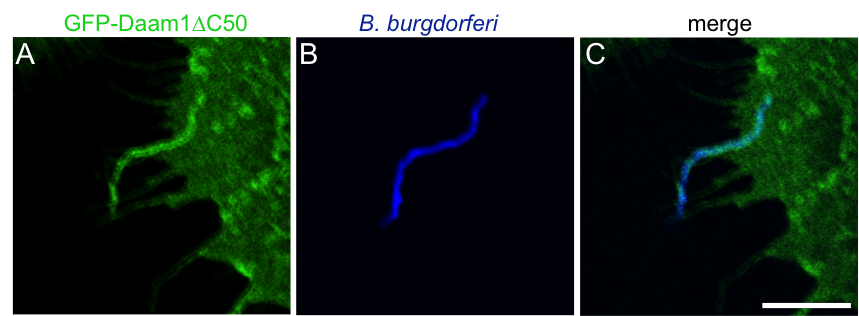
Enrichment of GFP-Daam1ΔC50 during phagocytosis of borreliae.
Confocal micrographs of primary human macrophage expressing GFP-Daam1ΔC50 (green; A) which is accumulated at the uptake structure of a Borrelia cell visualized by Hoechst 33342 staining of DNA (blue; B) with merge shown in (C). Scale bar: 5 µm
GFP-Daam1ΔC50 enrichment in uptake structure during internalization of Borrelia.
Time lapse movie shows primary macrophage expressing GFP-Daam1ΔC50 (green) capturing a Borrelia cell stained by Hoechst 33342 (blue). Note GFP-Daam1ΔC50 accumulations arising from the macrophage surface and closely following the sinuous morphology of the spirochete cell. Time since the start of the experiment is indicated in minutes. Scale bar 5 µm.
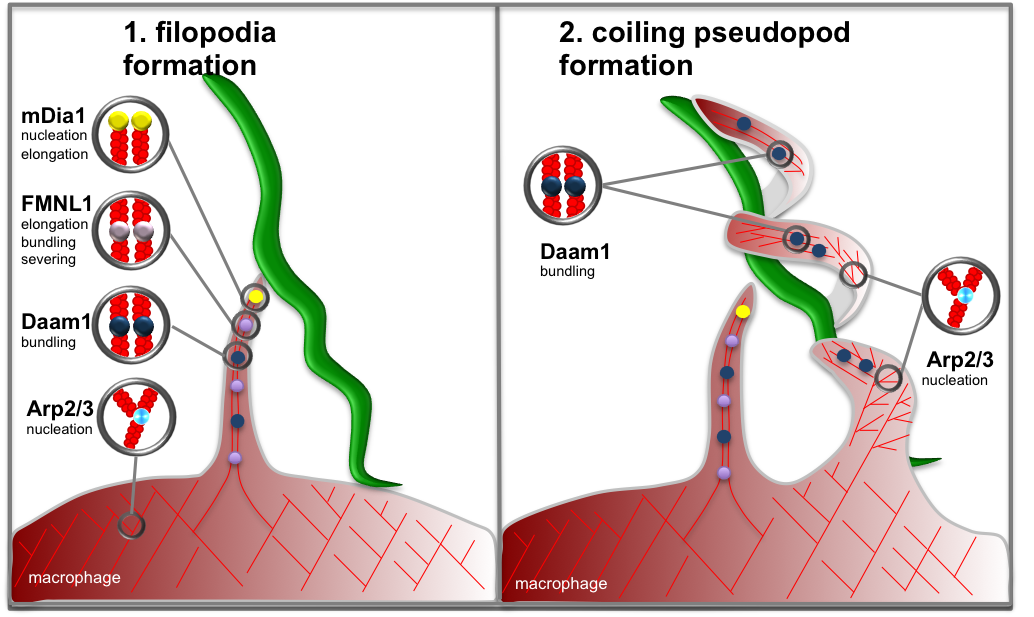
Model of formin- and Arp2/3 complex dependent actin regulation in coiling phagocytosis of Borrelia.
(LEFT) Upon stimulation with borreliae, macrophages form filopodial protrusions that arise from the cortical network. Filopodia are enriched in the formins mDia1 (localized at tips) and FMNL1 (localized at tips and shaft), which probably contribute to longitudinal growth of filopodia, and Daam1 (localized in filopodial shaft) which is probably involved through its actin-bundling activity. (RIGHT) Upon capturing of a Borrelia cell by filopodia, the spirochete is enwrapped by a coiling pseudopod. The flexibility of coiling pseudopods that enables them to enwrap the spiral-shaped borreliae is probably due to dot-like accumulations of Arp2/3 complex, which lead to formation of small branched actin networks and probably act as “hinges” at coiling nodes. Click image to enlarge
We could also identify the formin Daam1 as a further player in the regulation of phagocytosis of borreliae. Daam1 plays a dual role during Borrelia uptake, first as a regulator of Borrelia-capturing filopodia and second during the formation of the coiling pseudopod that enwraps the bacterial cell during internalization (Hoffmann et al., 2014). Knockdown experiments underline the requirement of all three formins for successful uptake of borreliae by primary macrophages. Until now, it was not clear whether coiling pseudopods develop from filopodia or if they constitute independent structures. Our live-cell experiments showed that Daam1-positive coiling pseudopods arise as a second independent structure from the macrophage surface and enwrap borreliae (Naj et al., 2013; Hoffmann et al., 2014; see model).
The role of RabGTPases Rab5a and Rab22a – and the role of compaction
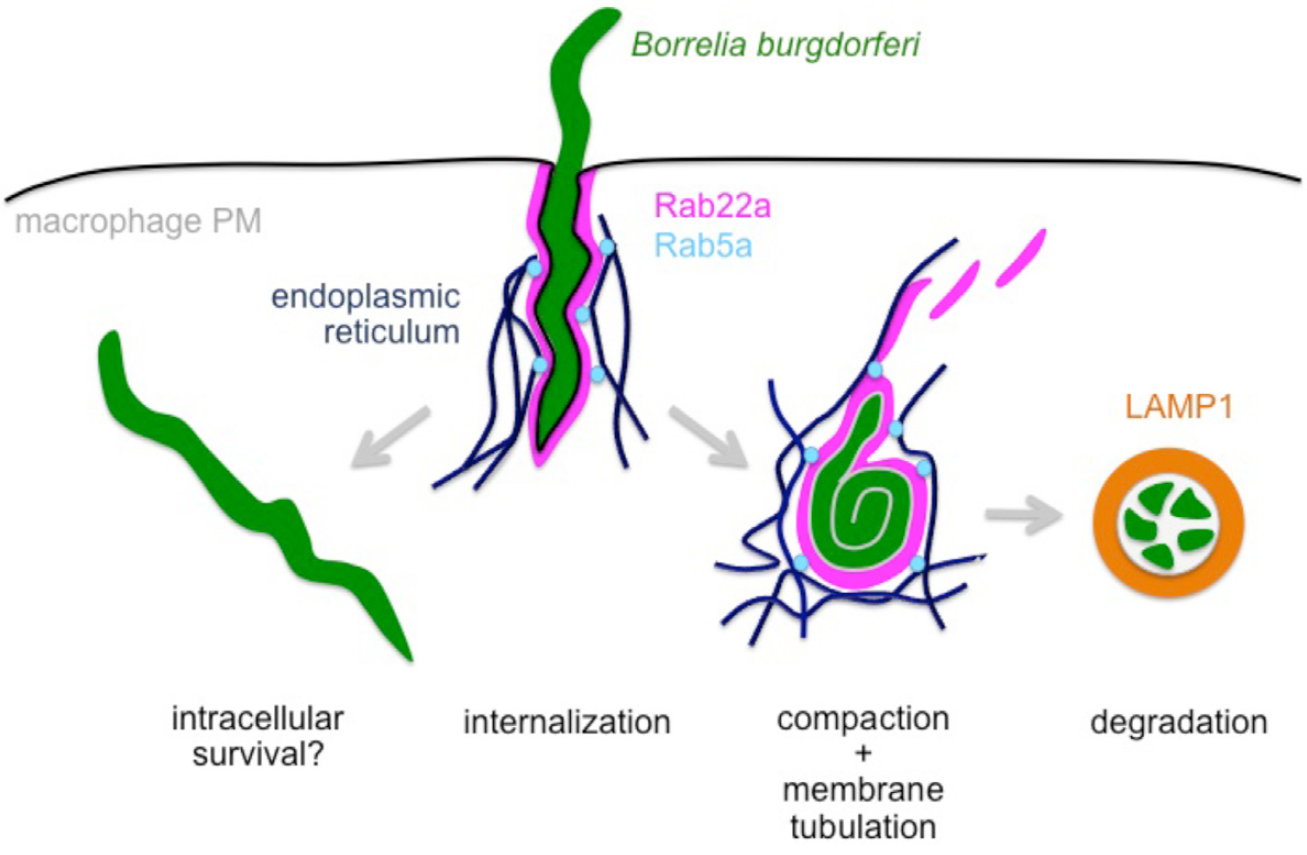
Model of RabGTPase-dependent intracellular processing of Borrelia burgdorferi by macrophages.
Borreliae captured by macrophages via coiling pseudopods are internalized through uptake into Rab22a-positive phagosomes. Phagosomes are subsequently contacted by Rab5a positive vesicles mediated by the endoplasmic reticulum. Subsequent membrane tubulation causes reduction of the phagosome surface, leading to visible compaction of borreliae. Further maturation of this compartment leads to its development into a degradative phagolysosome, indicated by the presence of the lyosomal marker protein LAMP1, and resulting in elimination of spirochetes. In contrast, escape from Rab22a-/Rab5a-dependent processing can lead to enhanced intracellular survival of borreliae. Click image to enlarge. (Naj & Linder 2015, J Cell Sci 12)
Intracellular trafficking by sorting nexins – SNX3 and Borrelia phagosome processing
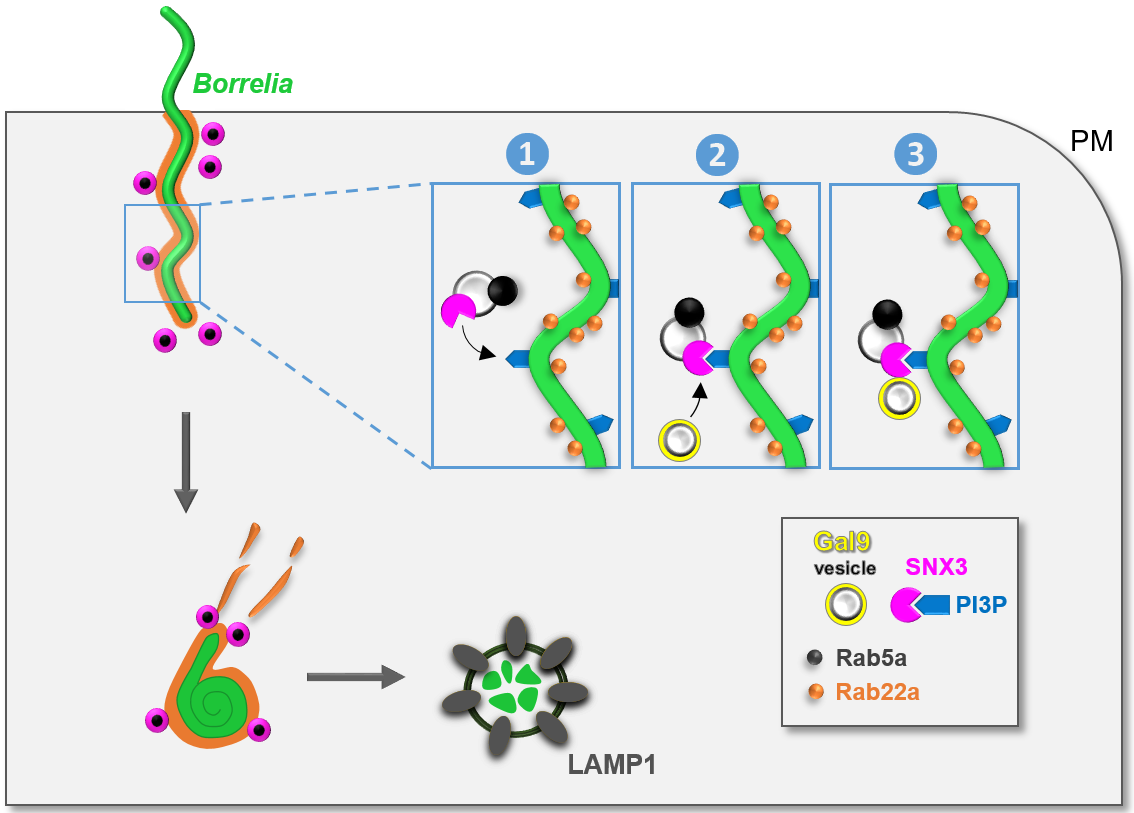
Model of SNX3-dependent processing of borreliae by macrophages.
B. burgdorferi is internalized by macrophages through uptake into Rab22a-decorated phagosomes that are contacted by Rab5a vesicles that also contain SNX3. SNX3 enables docking of Rab5a vesicles to phagosomes by binding to PI(3)P on the phagosomal surface (1), which is enriched at sites of high curvature due to the helical shape of the spirochete. SNX3 also recruits, by its C-terminal region, a second subset of vesicles positive for galectin-9 (2+3). Both Rab5a and galectin-9 contribute to phagosome compaction and phagolysosome maturation, leading to the elimination of spirochetes by macrophages. PM: plasma membrane. Click image to enlarge. (Klose et al. 2019, J Cell Biol 218).
It could be reported that previously found Rab5a vesicles carry sorting nexin 3 (SNX3), and that SNX3 binding to phosphoinositol-3-phosphate (PI(3)P), a phospholipid of the phagosomal coat, enables their tethering to borreliae-containing phagosomes. The SNX family comprises a heterologous set of proteins characterized by a phox (PX) domain, which enables binding to phospholipids, especially PI(3)P. We identified SNX3 as a regulator of phagosomal compaction, phagolysosome maturation, and intracellular processing of borreliae. siRNA-induced depletion of SNX3 led to a strong reduction in borreliae phagosomal compaction, which was rescued by expression of an siRNA-insensitive wildtype construct, but not by a mutant defective in PI(3)P binding. SNX3 depletion also led to reduced phagolysosomal maturation and increased intracellular survival of borreliae. Moreover, we identify galectin-9 as an interactor of the SNX3 C-terminus. Galectin-9 is a member of the galectin family that is known to bind carbohydrates on the cell surface but has also emerged as an intracellular regulator of protein and membrane trafficking pathways (Klose et al., 2019, see model).
Interaction of Borrelia burgdorferi with human macrophages – high resolution insights
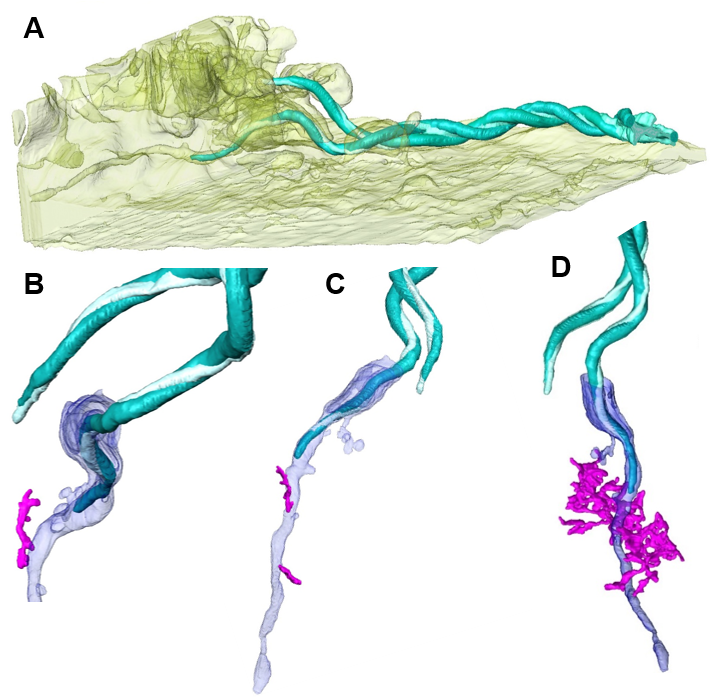
FIB/SEM-based renderings of captured Borrelia cell with associated membrane tunnel and ER contacts.
(A) Macrophage surface (yellow, semi-transparent) with associated and partially internalized Borrelia cell (turquoise). (B+C) Renderings of captured spirochete shown in (A), with associated membrane structures of the host cell (blue) and attachment site of the ER (magenta). (D) Different perspective of (B+C) with extended ER network. Click image to enlarge. (modified from Klose et al. 2020, J Cell Sci epub ahead of print)
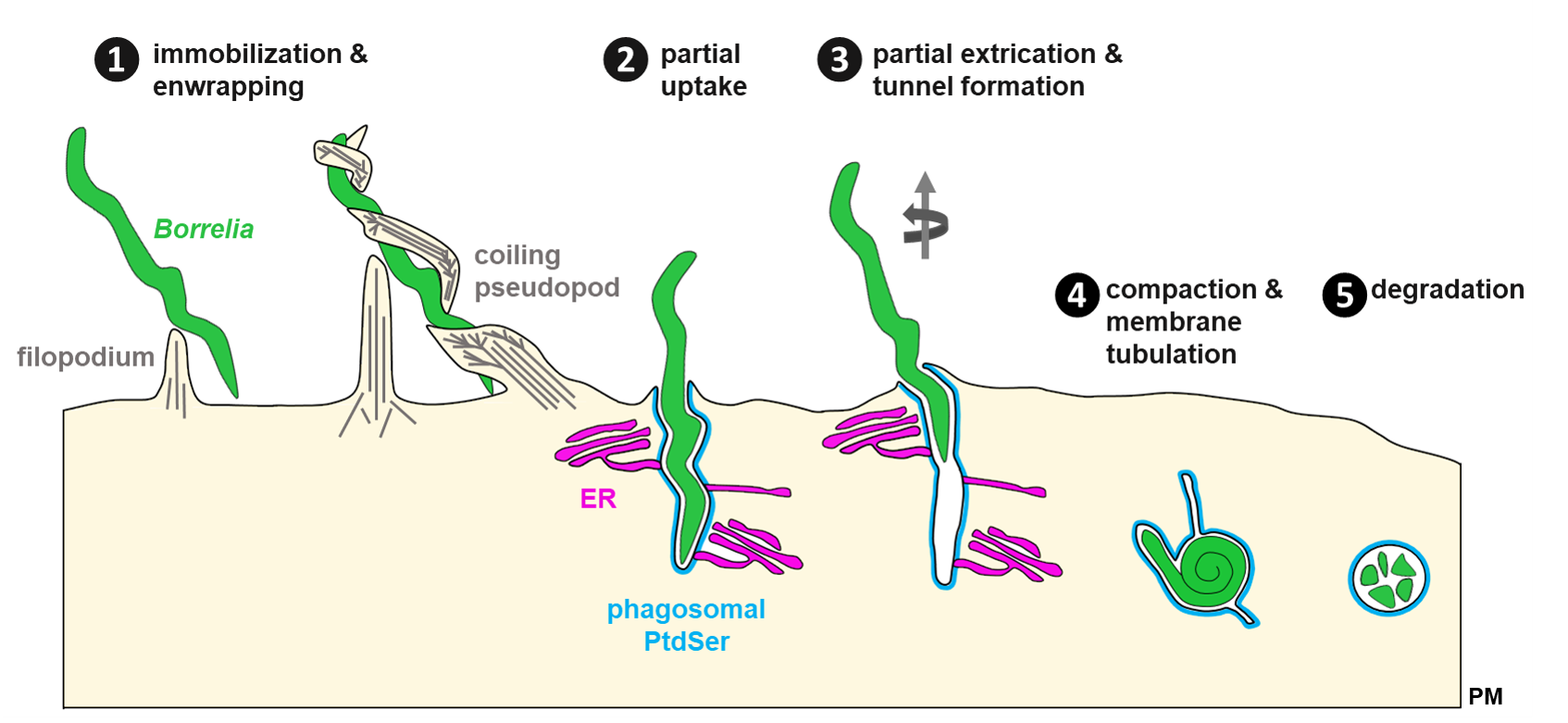
Model of Borrelia uptake and tunnel formation.
(1) Internalization of highly motile B. burgdorferi spirochetes by macrophages is mediated through contact of filopodiae and followed by enwrapment by a coiling pseudopod. (2) Contraction of the pseudopod leads to partial uptake of the Borrelia cell into the nascent phagosome positive for phosphatidylserine (PtdSer) that is contacted by the endoplasmic reticulum (ER). (3) Endoflagella-based motility of the extracellular part of the spirochete can lead to partial extrication of the spirochete and formation of an intracellular membrane tunnel which retains contact to the ER. (4) Membrane tubulation leads to compaction of spirochetes via loss of phagosomal surface. (5) Degradation of globular borreliae in mature phagolysosomes. PtdSer: phosphatidylserine; ER: endoplasmic reticulum; PM: plasma membrane. Click image to enlarge. (Klose et al. 2020, J Cell Sci epub ahead of print).
What’s next?
Current studies are focused on mechanisms driving the intracellular processing of borreliae by human macrophages and their phagolysosomal maturation including the mechanism of compaction.
related publications:
Woitzig P, Linder S. (2021)
Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages.
→ Biology (Basel) 2021 Jun 22;10(7):567. doi: 10.3390/biology10070567..
Klose M, Scheungrab M, Luckner M, Wanner G, Linder S. (2020)
FIB/SEM-based analysis of Borrelia intracellular processing by human macrophages.
→ J Cell Sci. doi: 10.1242/jcs.252320.
Klose M, Salloum JE, Gonschior H, Linder S (2019).
SNX3 drives maturation of Borrelia phagosomes by forming a hub for PI(3)P, Rab5a, and galectin-9.
→ J Cell Biol 218(9):3039-3059.
Naj X and Linder S (2017).
Actin-dependent regulation of Borrelia burgdorferi phagocytosis by macrophages.
→ Curr Top Microbiol Immunol 399:133-154.
Naj X and Linder S (2015).
ER-coordinated activities of Rab22a and Rab5a drive phagosomal compaction and intracellular processing of Borrelia burgdorferi by macrophages.
→ Cell Rep 12(11):1816-1830.
Hoffmann AK, Naj X, Linder S (2014).
Daam1 is a regulator of filopodia formation and phagocytic uptake of Borrelia burgdorferi by primary human macrophages.
→ FASEB J 28(7):3075-3089.
Naj X, Hoffmann AK, Himmel M, Linder S (2013).
The formins FMNL1 and mDia1 regulate coiling phagocytosis of Borrelia burgdorferi by primary human macrophages.
→ Infect Immun 81(5): 1683-1695.
Linder S, Heimerl C, Fingerle V, Aepfelbacher M and Wilske B (2001).
Coiling phagocytosis of Borrelia burgdorferi by primary human macrophages is controlled by CDC42Hs and Rac1 and involves recruitment of Wiskott-Aldrich Syndrome Protein and Arp2/3 complex.
→ Infect Immun 69(3):1739-1746.
last updated: 19-01-2021
