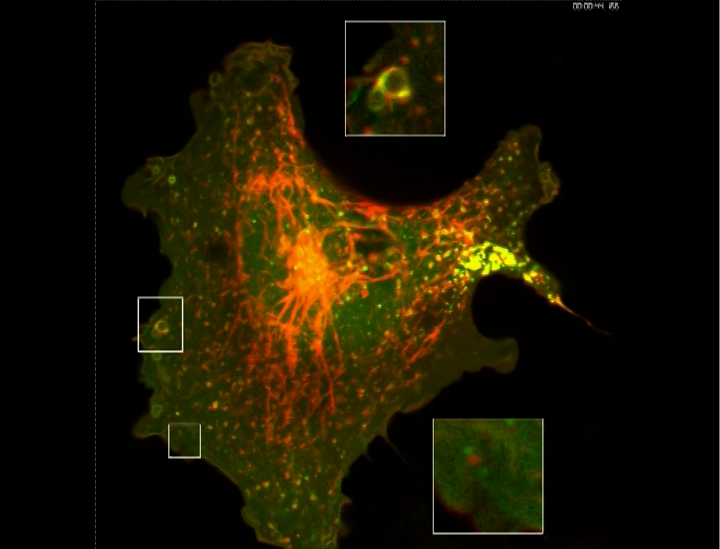
| Matrix metalloproteinases (MMPs) have emerged as major regulators of proteolytic cell invasion in various cell types. They can be separated into soluble and membrane type (MT) proteases. In particular, the membrane-bound isoform MT1-MMP (MMP14) is a key protease, which acts as a central regulator of proteolytic cell invasion in a variety of settings, including monocyte diapedesis, T-cell homing, and cancer cell metastasis. A complex regulatory network has evolved around MT1-MMP. Besides the activation of the protease by convertases and its inhibition by endogenous elements (e.g. Tissue Inhibitors of Metalloproteases (TIMPs)), the regulation also depends on multiple intracellular trafficking events. Surface-exposed MT1-MMP, in turn, can be internalized and recycled, a process that is regulated by both clathrin- or caveolae-dependent pathways. We investigate the role of kinesins and RabGTPases as motor proteins and regulators for the intracellular transport of MT1-MMP in primary human macrophages. We could already show that MT1-MMP positive vesicles travel bidirectionally along microtubules. This process is driven by kinesin-1 and -2 motors, as well as by cytoplasmic dynein, and regulates cell surface exposure of MT1-MMP (Wiesner et al., 2010). Furthermore, the impact of several specific RabGTPases on MT1-MMP trafficking and function in primary human macrophages could be demonstrated. The GTPases Rab5a, Rab8a and Rab14 critically regulate cell surface exposure of MT1-MMP, contact of MT1-MMP-positive vesicles with podosomes, as well as podosome-localised ECM degradation in 2D and 3D, and also 3D proteolytic invasion (Wiesner et al, 2013). Furthermore, our lab showed the importance of MT1-MMP islets underneath podosomes for their reemergence, highlighting the relevance of the protease for the main degradative structures of macrophages (El Azzouzi et al, 2016). Our current projects focus on a deeper understanding of the cellular processes MT1-MMP is involved in macrophages and how the proteases regulation is impacting general cellular mechanisms like migration, invasion and adherence. For this reason, we use different state of the art methods including 3D invasion assays, mass spectrometry, FACS analysis and 3D cancer cell co-invasion assays. |
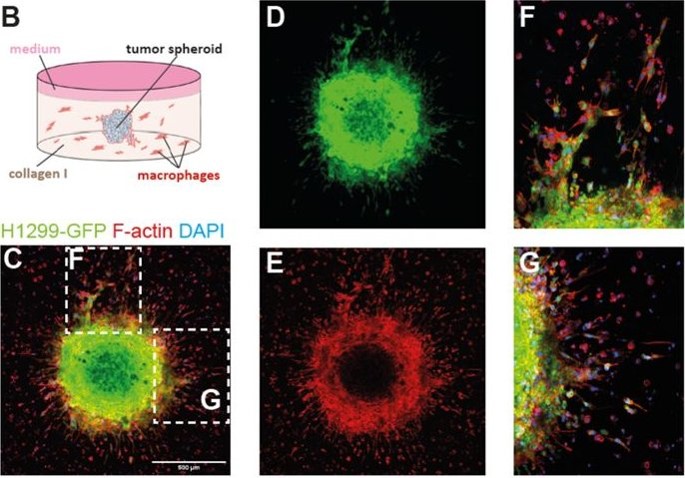
Co-invasion of H1299-GFP cancer cells and primary human macrophages.
Co-invasion of H1299-GFP cancer cells and primary human macrophages.
(B) Schematic drawing of tumor spheroid co-invasion assay. (C) Fluorescent image of a cancer cell spheroid (H1299-GFP) co-cultivated with primary human macrophages. The tumor cells can be identified by their GFP signal (D) and all cells are stained for F-actin (E). Detailed images from C (dashed white boxes) showing collective (F) and single cell invasion (G). (Hey et al, 2023)
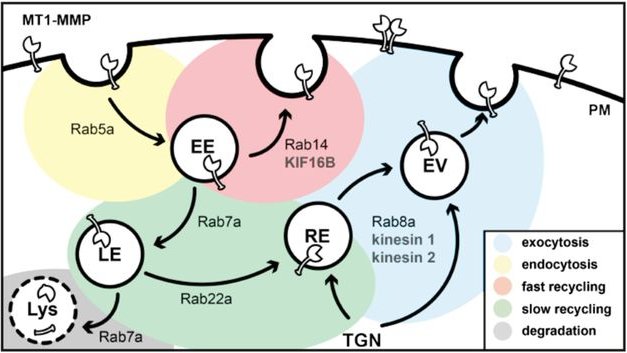
Model of MT1-MMP trafficking in primary human macrophages. Intracellular trafficking pathways to and from the plasma membrane are depicted, together with known vesicle regulators of the RabGTPase family and known kinesin motors associated with respective pathways. Note the role of KIF16B in the Rab14-dependent fast recycling pathway of the proteinase back to the cell surface. PM, plasma membrane; TGN, trans-Golgi network; EE, early endosome; EV, exocytic vesicle; RE, recycling endosome; LE, late endosome; Lys, lysosome. (Hey et al, 2023)